Heat Of Formation Table Salt
Table 1 summarizes our experimental data for pure salts as well as literature data. Hence the product is an instant hot compress If the salt is NH 4 NO 3 heat is absorbed when it dissolves and the temperature drops to about 0 for an instant cold pack.
Action Of Heat On Salts A Plus Topper
The potential utility of molten salts as heat transfer agents was also demonstrated for nuclear reactors as the liquid fuel in the Aircraft Reactor Experiment ARE and the Molten Salt Reactor.

Heat of formation table salt. Most metal carbonates decompose on heating to produce metal oxides and carbon dioxide gas. Compound Formula Compound Formula Calcium phosphate s 4132 CO aqueous unionized 41926 Calcium uoride s 12196 HCO 68993 Calcium hydride s 1862 Carbon trioxide 67523 Calcium hydroxide s 98609 Monatomic chlorine gas g Cl 12170. The molar heat of formation or standard enthalpy of formation is the change in enthalpy when 1 mole of a substance is formed from its elements under standard state conditions.
When a suitable reference state is chosen for each metal M and for each acid radical X and the heats of formation are referred to those states instead of the normal state the heat of formation per metal atom of any salt MX n is a constant times the valency of the metal M. 2 l acetic acid H aqC. 2-aq 086 kJmol C.
2 l methanoic acid H aqCHO. Effect of Heat on Chlorides. A salt is an ionic compound formed.
Most salt hydrate PCMs have moderate to high latent heats of fusion typically 100300 J g 1 124. Positive H heat adsorbed by the system from the surroundings The standard enthalpy of formation H0f of a compound is the change in enthalpy that accompanies the formation of 1 mole of a compound from its elements with all substances in their standard states. FH Standard molar enthalpy heat of formation at 29815 K in kJmol fG Standard molar Gibbs energy of formation at 29815 K in kJmol S Standard molar entropy at 29815 K in Jmol K Cp Molar heat capacity at constant pressure at 29815 K in Jmol K The standard state pressure is 100 kPa 1 bar.
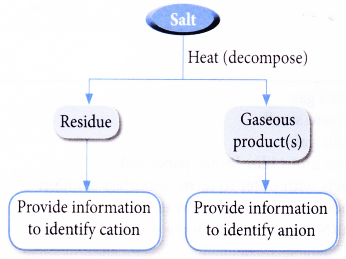
Some heats of solutions and heats of hydration for dilute solutions in pure water at 15 C. 1 The nomenclature of heat stable salts is somewhat confusing. Table shows the action of heat on carbonate salts.
Metal carbonate metal oxide carbon dioxide. Table of Solubility of Salts. If the treating solution is contaminated by organic acids these acids sometimesare called heat stable salts.
Standard Enthalpy of Formation for Atomic and Molecular Ions Cations H f kJmol Cations H f kJmol Anions H f kJmol Anions H f kJmol Agaq 1059 Kaq 2512 Braq 1209 H 2PO 4 aq 13025 Al3aq 5247 Liaq 2785 Claq 1674 HPO 4 2aq 12987. In an MEA process when the contaminants COS and CS 2 are present in the acid gas stream an undesirable side reaction occurs resulting in the formation of heat-stable salts. Solute Products Heat of solution EXOTHERMIC CH.
2-aq 15 kJmol CH. If the amine is purposely neutralized the protonatedamine is termed the heat stable salt. The first challenge is that some salt hydrates melt incongruently.
When the carbon dioxide gas is bubbled through limewater it will turn the limewater milky. The heat of reaction is then minus the sum of the standard enthalpies of formation of the reactants each being multiplied by its respective stoichiometric coefficient plus the sum of the standard enthalpies of formation of the products each also multiplied by its. From this empirical rule the heat of solution can be calculated when the heat of formation in the solid state is known.
The heat of fusion for pure salts excluding calcium nitrate can be found along the y-axis at 0 mol Ca. Upon heating a portion of the salt hydrate dehydrates to a less hydrated phase before melting. The steam released comes from the hydrated water of the crystallize salt.
Free energy of formation at 1000 K of some alkali fluorides and transition metal. Standard heats of formation of selected compounds. Due to side reactions andor degradation a variety of contaminants will accumulate in an amine system.
Action of Heat on Carbonate Salts. If the salt is CaCl 2 heat is released to produce a solution with a temperature of about 90C. The method of removing these depends on the amine involved.
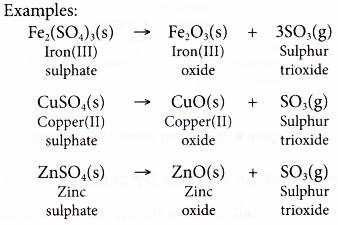
Oaq 02 kJmol CaCl. In this paper HSS refers to the thermally-unregenerable protonated amine. 2FeSO 4 7H 2 O Fe 2 O 3 p SO 2 g SO 3 g 14H 2 Og Meanwhile zinc sulphate copper II sulphate and iron III sulphate decompose when heated strongly to evolve sulphur trioxide gas and form a metal oxide.
The standard enthalpy change of formation is the sum of the heats of formation of the products of a reaction minus the sum of the heats of formation of the reactants. You need to know the values of the heat of formation to calculate enthalpy as well as for other thermochemistry problems. Table of Some Double Salts.
43 rows Also called standard enthalpy of formation the molar heat of formation of a compound H f is equal to its enthalpy change H when one mole of a compound is formed at 25 degrees Celsius and one atom from elements in their stable form. A given reaction is considered as the decomposition of all reactants into elements in their standard states followed by the formation of all products. While salt hydrates are very attractive PCMs from the standpoint of energy storage density they do have some shortcomings 126.
Effect of Heat on Salts. Effect of Heat on NitrateV Effect of Heat on SulphateVI Effect of Heat on CarbonatesIV and Hydrogen CarbonateIV. These salts should be removed from the system.
219 rows These tables include heat of formation data gathered from a variety of sources including. 2 aq 2Cl-.

Enthalpy Heat Combustion Experiment 4 Heat Exothermic Reaction Energy Level

The Science Of Salt Mps Property Services

Heat Of Formation Thermodynamics Chemistry Khan Academy Thermodynamics Khan Academy Chemistry

Table Salt Can Be Poisonous The Times Of India

Brax Toss In A Bit Of Borax Sodium Tetraborate For Green Flames Water Sftener Salt Chuck In Some Water Softener Salt For Purple Flames Tablesalt A Dash Of Water Softener Salt

Calorimetry 2 2 Chemistry Thermodynamics Teaching
Action Of Heat On Salts A Plus Topper

Total 2 Average 5 X2f 5 What Is Enthalpy Of Reaction Heat Of Reaction The Amount Of Heat Given Out Or Abso Molar Mass Reactions Chemical Equation

Making Table Salt Using Sodium Metal And Chlorine Gas Youtube

Reaction Of Nacl Sodium Chloride And Agno3 Silver Nitrate Then Ammonia Silver Chloride Chemical Formula Chemical Reactions

Determined To Succeed Teaching Chemistry Chemistry Education Chemistry Classroom

The Science Of Salt Mps Property Services

Chemistry 10 5 Heat Of Reaction Chemistry 10 Chemistry Chemical Equation





Posting Komentar untuk "Heat Of Formation Table Salt"